Confocal Microscopy
저자: Maura Francis, Cory Boone, Jaclyn Wycoff
Confocal microscopy is a type of fluorescence microscopy which uses a laser to excite fluorescence from fluorophores used to label different subsets of a specimen. Fluorescence microscopes are used for imaging cells and tissues that have been fluorescently tagged.1 What separates confocal microscopy from conventional epi-fluorescence microscopy is its increased resolution when imaging thick specimens, elimination of out-of-focus glare due to spatial filtering, and reduction of light-induced damage to the sample, known as phototoxicity.
Instead of using an incoherent tungsten or mercury lamp source like conventional microscopes, confocal microscopes use a laser to illuminate the sample.1 The system then collects images at planes at various depths in the sample, known as optical sections, by scanning the laser through different focus positions.1 Optical sections enable live specimens to be imaged because the sample doesn’t need to be physically sectioned off. The illumination of smaller sections also helps specimens to remain viable longer by significantly reducing the effects of phototoxicity. The amount of light that conventional techniques use for illumination makes it difficult to ensure the vitality of the specimen imaged, which is crucial when capturing biological events.1
Image Appearance
When compared to images captured by a conventional fluorescence microscope, images from a confocal microscope system exhibit a marginal increase in axial and lateral resolution, but spatial filtering from the system’s pinhole assembly blocks out-of-focus fluorescence for a much more detailed image (Figure 1).2 Contrast of the image is improved over traditional methods due to a reduction in background noise and improved signal-to-noise ratio. When using conventional techniques, the fluorescence emitted from adjacent parts of the sample interferes with the image plane and obscures the focus.1 This is problematic because there is no way of differentiating the fluorescence that does not contribute to the focal plane being imaged, which is especially apparent in specimens that are thicker than two micrometers.1 By utilizing spatial filtering techniques, confocal microscopes are able to eliminate this problem by blocking any out-of-focus light that would otherwise obscure the image.2 Confocal and multiphoton microscopy have similar resolution in the x- and y-axes, but multiphoton microscopy has superior resolution in the z-axis, or depth. Compared to multiphoton microscopy, confocal microscopy only uses a single photon to excite fluorescent dyes, which can result in more light scatter in the image and the inability to image as deep into a sample. Confocal techniques commonly use light from the visible spectrum, which also tends to scatter and absorb more in biological tissue than longer wavelengths, which in turn can limit the possible penetration depth into a sample. However, the confocal technique still has proven to have enhanced resolution over traditional widefield techniques, allowing it to be considered a bridge between classic widefield methods and more advanced super-resolution techniques. Confocal microscopy is very well-suited for imaging of 2D cell cultures while multiphoton and light sheet microscopy provide advantages for imaging 3D samples.
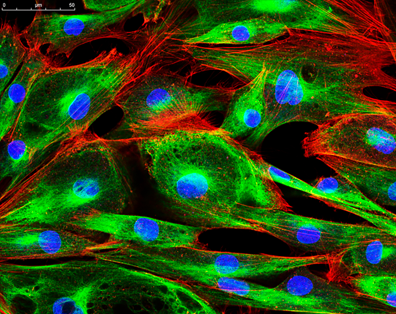
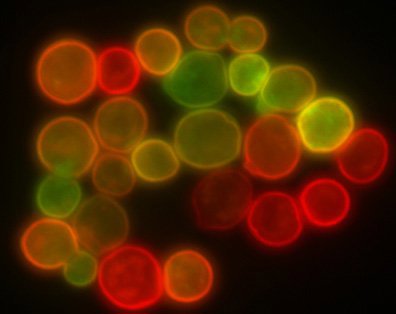
Figure 1: An image of protoplasts captured using confocal microscopy (left) that is more finely focused than an image of microspheres of a similar size captured using conventional epi-fluorescence microscopy.
Technical Details
Figure 2 depicts the typical layout of a confocal microscope in which there are four key components:
- Laser Excitation Source: emits a laser beam into the system to focus on the sample.
- Fluorescence Dichroic Filters: reflects the excitation light onto the specimen and passes the secondary fluorescence, given off by the specimen, through to the detection system.
- Pinhole: an essential component of confocal setups, it acts as a spatial filter. It prevents any light that is not confocal to the objective focal plane from interfering with the image.2
- Stepper Motors: allows the laser beam to travel incrementally across the sample, obtaining x and y scans collected along the z-axis. This automates three-dimensional data being collected, making it easy to create a 3D image.2
- Objective: a high numerical aperture (NA) objective is typically used to achieve high resolutions. Water or oil immersion designs are often used to adjust for refractive index mismatch between the aqueous medium many live samples are kept in. Long working distances are required for thick specimens and 3D imaging/optical sectioning.

Figure 2: Schematic of a typical confocal microscopy system
Comparison to Other Microscopy Techniques3
| Technique | Resolution | Sample Size | Relative Cost | Photobleacing |
|---|---|---|---|---|
| Confocal Microscopy | <µm | µm | $$ | Yes |
| Multiphoton Microscopy | <µm | mm | $$$ | Less |
| Light Sheet Fluorescence Microscopy | µm | >cm | $ | Least |
Confocal Microscopy at Edmund Optics®
Edmund Optics® supplies a wide range of optics for confocal fluorescence microscopy applications including filters, objectives, laser systems, and pinholes.

 Fluorescence Filter Sets
Fluorescence Filter Sets
- Excitation, Emission, and Dichroic Filters for Fluorescence Imaging
- >93% Transmission and OD 6 Blocking for Maximum Brightness and Contrast
- Hard Sputtered Coatings on Single Substrate
BUY NOW

 Pre-Mounted Fluorescence Filter Cube Sets
Pre-Mounted Fluorescence Filter Cube Sets
- Ensure Proper Alignment of Filter Sets
- Easily Adapt to Common Microscopes
- Available in a Variety of Wavelengths
BUY NOW

 Fluorescence Bandpass Filters
Fluorescence Bandpass Filters
- Common Wavelengths for Popular Fluorophores
- Excitation Filters and Emission Filters Available
- >93% Transmission
- >OD 6 Blocking, <3% from Edge of Bandwidth
BUY NOW
 High Performance Fluorescence Dichroic Filters
High Performance Fluorescence Dichroic Filters
- Improved Flatness, Transmitted Wavefront, and Surface Quality
- Ideal for Fluorescence Microscopy or High Magnification Imaging Applications
- Most Popular Cut-On Wavelengths of Fluorescence and Longpass Dichroic Filters
BUY NOW

Olympus Water Immersion Objectives
- Water Immersion Objectives with Magnification Ranges from 10 to 40X
- Displays Flat Images from High Transmission Factors up to the Near-Infrared Region of the Spectrum
- Ideal for Fluorescence Imaging of Tissue and Specimens, such as Brain Tissue
BUY NOW

Olympus X-Line Extended Apochromat Objectives
- High Numerical Aperture (NA) up to 1.45
- Chromatic Aberration Correction from 400 - 1000nm
- Uniform Image Flatness over Large FOVs
BUY NOW

Mitutoyo Infinity Corrected Long Working Distance Objectives
- Long Working Distances
- Bright Field Inspection
- High Quality Plan Apochromat Design
- Flat Image Surface over Entire Field of View
BUY NOW

Coherent® High Performance OBIS™ LX/LS Laser Systems
- Same Compact Design for All Wavelength Options
- Integrated Control Electronics with Analog and Digital Modulation
- Circular Beam with Superior Beam Quality
BUY NOW
Coherent® High Performance OBIS™ LX/LS Fiber-Pigtailed Laser Systems
- High Performance OBIS™ LX/LS Lasers with Added Fiber Optic Capability
- Permanent Fiber Attachment Extends Lifetime with Guaranteed Power
- Single-Mode Polarization-Maintaining Fiber with an FC/APC Connector Provide High-Quality and Low-Noise Laser Beam Output
BUY NOW

Precision Pinholes
- Available in Aperture Mounts for a Secure Mechanical Support
- Pinhole Sizes Ranging from 1 to 1,000 Microns
- High Power Apertures Available
BUY NOW

Acktar Blackened Pinholes
- Low Reflectance from the EUV to SWIR
- 99% Absorbance Blackening Coating
- Aperture Sizes from 20 to 1000μm
- Available Unmounted and Mounted
BUY NOW
References
- Paddock, Stephen W., et al. “Introductory Confocal Concepts.” Nikon, https://www.microscopyu.com/techniques/confocal/introductory-confocal-concepts.
- Fellers, Thomas J., and Michael W. Davidson. “Introduction to Confocal Microscopy.” Olympus Scientific Solutions Americas Corp., https://www.olympus-lifescience.com/ru/microscope-resource/primer/techniques/confocal/confocalintro/.
- Santi, P. A. (2011). Light Sheet Fluorescence Microscopy. J Histochem Cytochem, 59(2), 129-138. doi: 10.1369/0022155410394857.


























본사 및 지사별 연락처 확인하기
견적 요청 도구
재고 번호 입력 필요
Copyright 2023, 에드몬드옵틱스코리아 사업자 등록번호: 110-81-74657 | 대표이사: 앙텍하우 | 통신판매업 신고번호: 제 2022-서울마포-0965호, 서울특별시 마포구 월드컵북로 21, 7층 (서교동, 풍성빌딩)